It occurs world-wide and is responsible for 95% of cases of hepatitis previously classified as non-A, non-B hepatitis. It belongs to the Hepacivirus, a member of the Flaviviridae virus family.
There are at least seven subtypes of hepatitis C, each responding differently to treatment. The most common hepatitis C genotypes found in Australia are type 1 (52%), type 3 (32%), type 2 (9%) and type 4 (5%).
Hepatitis C is usually spread from person to person by the passage of blood through the sharing of injecting equipment among drug users, tattooing, body piercing, needle-stick injuries, unsafe handling of blood products, blood transfusion or from mother to baby. Healthcare personnel and laboratory staff handling blood and blood products are at increased risk.
Hepatitis C virus is predominantly a blood-borne virus, with a very low risk of sexual or vertical transmission in heterosexual contact, but a much higher rate of transmission in MSM sexual contact. The incidence in Australia of new infections has declined since 2000.
Structure
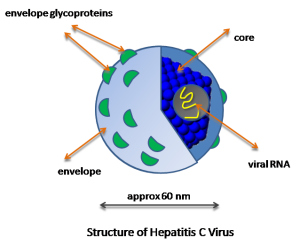
The hepatitis C virus particle consists of a core of genetic material (RNA), surrounded by an icosahedral protective shell of protein, and further encased in a lipid (fatty) envelope of cellular origin. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope.
About 20-30% of people with chronic hepatitis C infection will develop cirrhosis, generally after 20-30 years of infection.
Features
Patients with hepatitis C usually have no symptoms in the early stages (10-15% are acutely jaundiced), and many remain asymptomatic. All patients testing positive for the virus should be considered infectious. About 75% become chronic carriers of the virus and a proportion of these develop cirrhosis and cancer. 25% of people infected will clear the virus (although still carrying antibodies).
A seroconversion window period ranging from two to twenty-six weeks may delay the production of antibodies.
In Australia some 225,000 people are infected with hepatitis C and about 9,700 new cases are notified each year.
School exclusion
Children with hepatitis C are not excluded from school.
In Victoria, hepatitis C is a Group B notifiable disease and must be reported within 5 days.
Transmission of hepatitis C in Australia
HCV is usually spread by blood or blood contaminated body fluids by:
– sharing needles or other equipment (60%)
– blood transfusion or the receiving of blood products (20%)
– tattooing, acupuncture, blood spread from razor blades, tooth brushes, nosocomial and occupational exposure (5%)
– no known risk factor (15%). (Many of these patients have been born overseas in areas such as the Mediterranean, the Middle East, South America and South-East Asia, where higher rates of endemic HCV infection and historically unsterile medical practices may be significant in causality.)
Hepatitis C is not regarded as a sexually transmitted infection in Australia and the incidence of heterosexual transmission in a patient with chronic HCV infection is very low. According to Hepatitis Australia, transmission during sex occurs only when infected blood is present.
Similarly, transmission from mother to baby is very low (approx 6%), unless other diseases such as HIV are also present. Hepatitis C positive mothers should breastfeed their babies unless nipples are cracked or bleeding.
Over 2000 cases were notified in Victoria during the first 9 months of 2006.
International scene
About 3% of the worlds population has hepatitis C. Many African countries have rates exceeding 10% and Egypt has a rate of about 20% (following public health measures to control schistosomiasis, involving multiple injections in the 1950s – 1980s).
Hepatitis C is not a risk for the usual international traveller.
Vaccine Information.
There is no vaccine available for hepatitis C, but a new therapeutic vaccine is currently being trialled. Chiron Corporation is combining its recombinant cocktail with CSLs iscomatrix adjuvant in Phase II trials in Australia.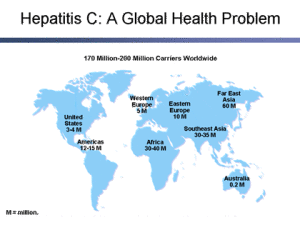
Recommendations for post-exposure interventions to prevent infection have been prepared by CDC (2008).
Management
All people with risk factors for hepatitis C should have a serological screening test for anti-hepatitis C antibodies.
A positive screening test should be followed by a test for hepatitis C RNA to confirm the diagnosis.
The hepatitis C genotype and viral load should then be determined.
The severity of fibrosis should be assessed by clinical and laboratory assessment and the use of non-invasive serum scores.
Transient elastography is particularly recommended when serum scores do not clearly exclude cirrhosis. Patients with a high likelihood of cirrhosis should be managed in a specialist setting.
Patients with chronic hepatitis C should be treated with oral direct-acting antivirals. The treatment regimen and duration should be selected according to hepatitis C genotype, viral load, previous treatment experience and the presence or absence of cirrhosis.
Adherence to the antiviral regimen is essential. To establish whether treatment was successful, patients should be tested for hepatitis C RNA 12 weeks after completing treatment.
1. Patients with hepatitis C are encouraged to acquire vaccination with both the hepatitis A and hepatitis B vaccines
2. Reduce the risk of contracting hepatitis B or HIV
3. Reduce the risk of transmission e.g. sharing of razor blades and tooth brushes should be discouraged
4. Limiting alcohol intake to 10 g/day
5. Antiviral treatment should be considered in all patients with hepatitis C, see below.
Recent improvements in treatment have revolutionised the management of hepatitis C.
New guidelines for pre-treatment and treatment protocols are available at the Australian Prescriber.
History
Investigators from the Centers for Disease Control (headed up by Daniel W. Bradley) and Chiron (Michael Houghton) identified the virus in 1989. In 1990, blood banks began screening blood donors for HCV, but it wasn’t until 1992 that a blood test was perfected that effectively eliminated HCV from the blood transfusion supply.